Electrochemical cells and batterries

Electrochemical cells and batteries
In Chapter 7, you used cells such as the one in Figure 2 below.

The cells have a positive terminal and a negative terminal. The positive terminal is the knob on the top of the cell and the negative terminal is the flat end of the cell. The terminals are marked + for positive and − for negative. Find the + and − marks on the cell or battery you have.
The voltage of a cell is also shown on it. Find the number on the cell or battery you have. It will be 1,5 V or 9 V. The voltage is the amount of energy that the cell can give to the electricity.
In Natural Sciences this year, you will learn about chemical reactions. An "electrochemical cell" uses chemical reactions between substances inside the cell to give energy to electricity.
What is inside a cell?
You can buy two types of electrochemical cells. The pictures below are called "cut-away diagrams". The cell is drawn as if the outer covering has been cut away to show you the inside.
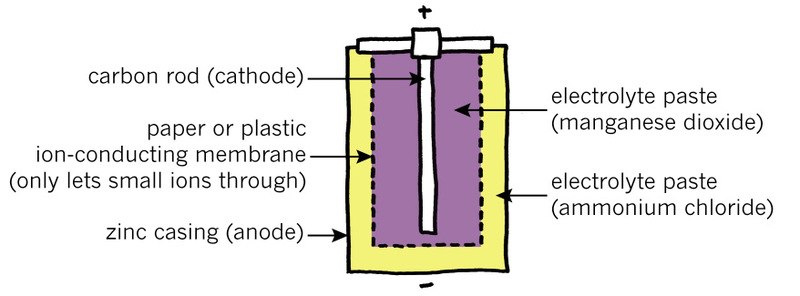
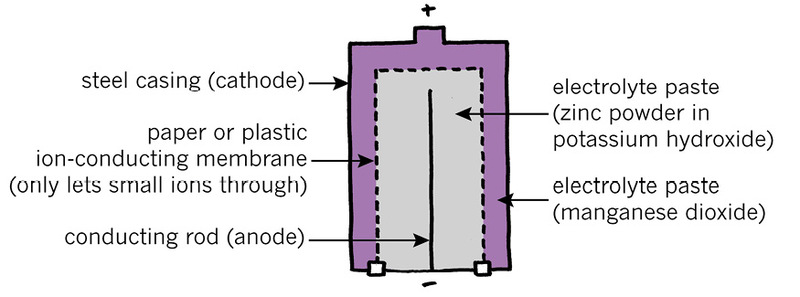
The zinc-carbon cell in Figure 3 is a less expensive kind of cell that does not last as long as an alkaline cell. Both types of cells have a "positive electrode" and a "negative electrode", and these electrodes are in a syrupy substance called an "electrolyte paste."
The electrolyte in the alkaline cell contains potassium hydroxide, which is an alkali. This is the reason for the name of the cell.
In the zinc-carbon cell, the negative electrode is made of zinc metal. This zinc has been shaped into the casing that contains the paste of electrolyte. Outside the zinc casing is a thin steel casing, which prevents you from seeing the zinc.
1. Which part of the zinc-carbon cell is the positive electrode?
In the alkaline cell in Figure 4, the steel casing is the positive electrode. The knob on the top of the cell is part of the casing. The casing is usually wrapped in plastic, except for the knob at the top.
2. Which part of the alkaline cell is the negative electrode?
Make a cell and a battery
This activity has two parts. First, each team in the class will make one cell. Then all the teams will connect their cells to make a battery and light a light bulb.
Each team needs:
- two 5-cent coins or pieces of copper of about the same size,
- a galvanised metal washer, which is a disc with a hole in the middle,
- a piece of cloth or cardboard
about the same sizeor slightly smaller than the 5-cent coin,
soaked in salty water,
Galvanised means it's coated with zinc.
- a piece of cooking foil, about the size of two fingers next to each other, and
- sticky tape.
Your teacher needs:
- a voltmeter or multimeter,
- a bowl of salty water − 1 teaspoon of salt to 100 ml of water,
- a light bulb,
- a beeper that will work at 3 volts, and
- six crocodile-clip wires, three insulated with red plastic and three with black plastic.
Part 1: Make your cell
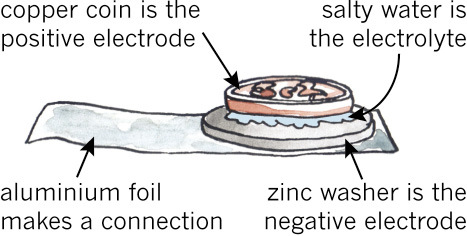
- Fold the cooking foil lengthwise, so that you have a long piece that is double in thickness. Put it on the table. The foil is made of aluminium, which is a good conductor.
- Squeeze the salty water out of the cloth.
- Next, put the zinc washer on the foil, put the wet cloth on the washer, and put the copper coin on top of the wet cloth.
- The cloth must not hang over the zinc washer, and the salt water must not run down the sides of the coin and washer. If this happens, it will create a short circuit between the copper and zinc, which you don't want.
The zinc washer is your negative electrode, the copper coin is your positive electrode, and the salt water is your electrolyte.
Now you have made a cell. The cooking foil is the negative terminal where you can connect the voltmeter.
Call your teacher to measure the voltage!
Part 2: Make a battery
Each team should bring their cells to the front table and connect the cells as seen in Figure 6 below.
You will connect six cells, but Figure 4 only shows four cells, to make the picture easier to understand.
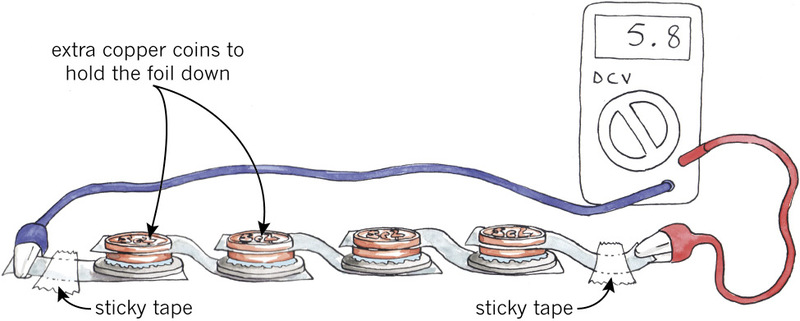
You have two 5-cent coins. The first one is the positive electrode of the cell, and the second 5-cent coin is there to press the foil down on the first coin to make good contact.
Use sticky tape to hold the cooking foil down on the table, and connect wires to the ends. The ends of the foil are your terminals.
Your teacher will measure the voltage of the whole battery. With six cells, the voltage of the battery will be about 6 volts or slightly less.
Now connect a light bulb to the positive and negative terminals of the battery. Does the light bulb glow?
Connect a beeper to the positive and negative terminals. Remember to connect the red wire to the positive terminal. Can you hear it beep?
1. What are the two different metals used for the positive and negative electrodes?
2. How is the voltage of the battery different from the voltage of one cell?
Batteries do not provide the full amount of volts
Before you connect the bulb or beeper, the battery has energy but it is not producing a current, and its voltage is about 5,8 V. As soon as you connect the bulb or beeper and the battery makes a current flow through the circuit, the voltage drops to about 1,8 V. This happens because the current loses a bit of its energy in the battery as it passes through the salt water and all the connections at the electrodes. You call this the "internal resistance" of the battery.
Rechargeable batteries
Car batteries are rechargeable
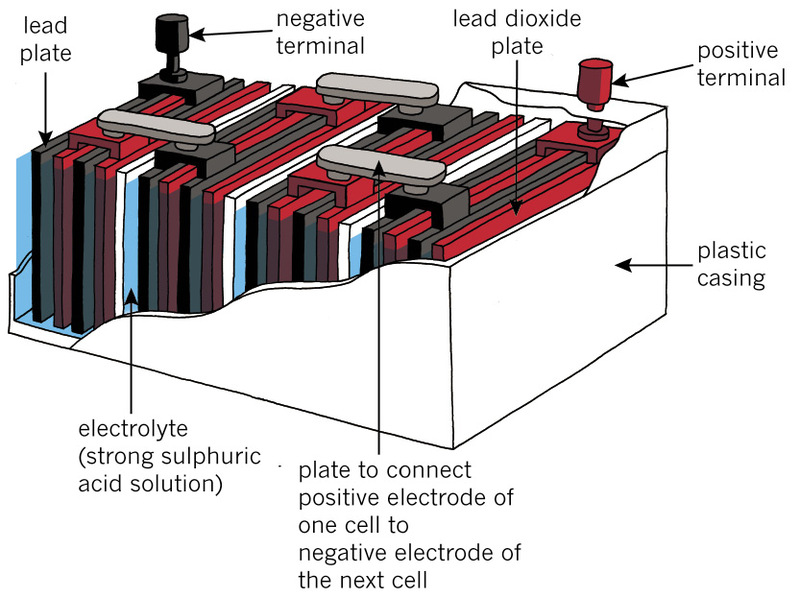
Figures 7 and 8 show the inside of a car battery. The colour red is used to show the positive electrodes, and dark grey is used to show the negative electrodes. The colour blue is used to show the liquid electrolyte. The electrodes and electrolyte do not really have these colours. Everything inside a battery looks mostly grey.
A car battery has six cells and it can give energy at 12 volts. To keep the diagram in Figure 7 simple, only four cells are shown.
1. What kind of diagram is Figure 7?
2. What is the positive electrode in each cell made of?
3. What is the negative electrode made of?
4. What kind of electrolyte is between the electrodes?
5. Are the cells in a car battery in series or in parallel?
6. A car needs 12 volts and a very big current to turn the starter-motor and start the engine. Sometimes, on cold mornings, a car won't start. A mechanic can test the battery, and might say "There is one dead cell in this battery". If the battery has a dead cell, what voltage will the battery give?
7. On a voltmeter, the battery might show that it will give 12 volts, but when you try to start the car, it won't start. Give a possible reason why the battery is not strong enough to start the car.
8. What can you measure to test your idea?
A car battery is different to the cells and batteries we usually buy. When we have taken all the energy from the battery, we can recharge the battery and give it energy again. A motor car has a "generator" or "alternator" that takes energy from the engine and gives it to the battery while you drive the car. You will learn about generators in the next chapter. A cell-phone battery is also a rechargeable battery.
Batteries with cells in series or in parallel
In Chapter 7, you learnt about connecting bulbs in series or in parallel. You can also connect cells in series or in parallel. You might get confused between "bulbs in series" and "cells in series". Look at the two figures below.
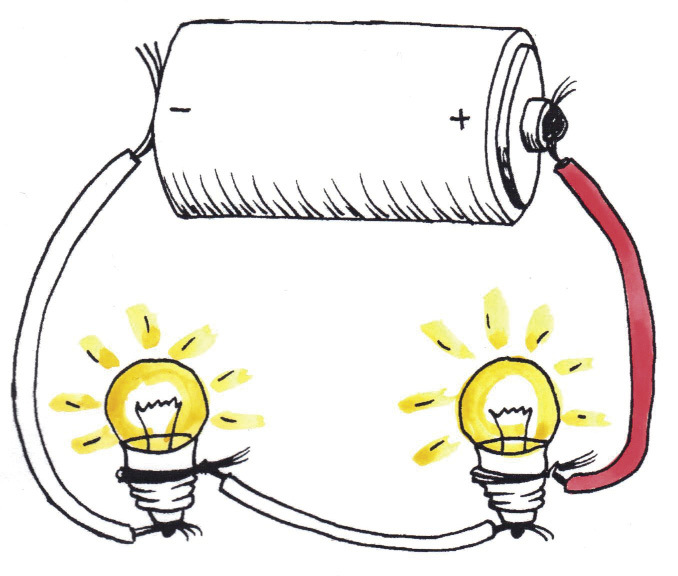
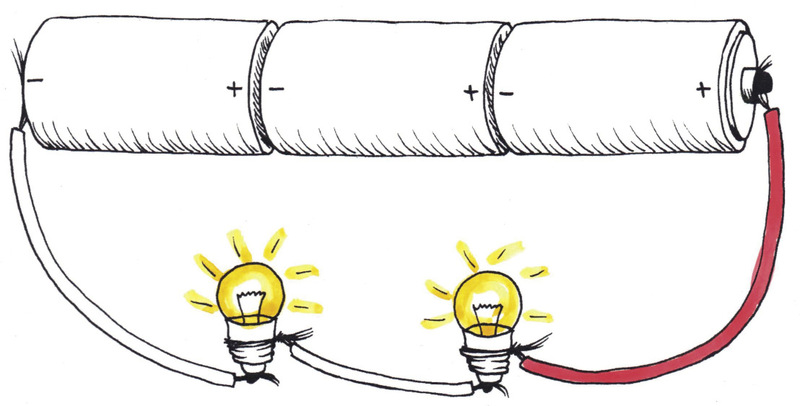
The bulbs in Figure 10 glow brighter than those in Figure 9 because they share the 4,5 V from the cells in series, so they get 2,25 V each. The bulbs in Figure 9 share the 1,5 Volts from one cell, so they get only 0,75 V each.
You might also get confused between "bulbs in parallel" (in Chapter 7) and "cells in parallel".
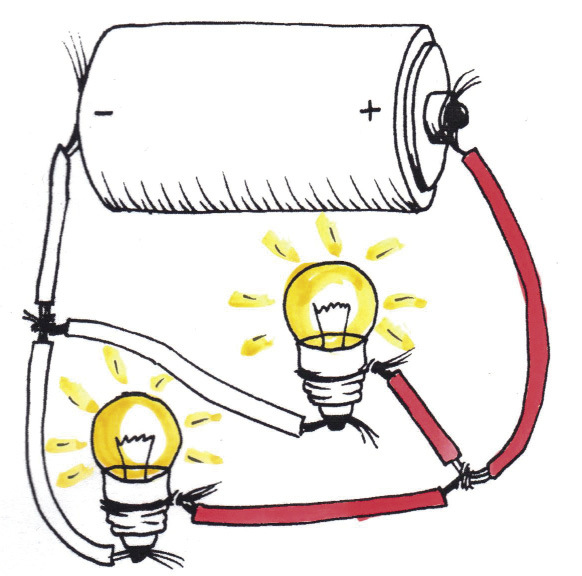
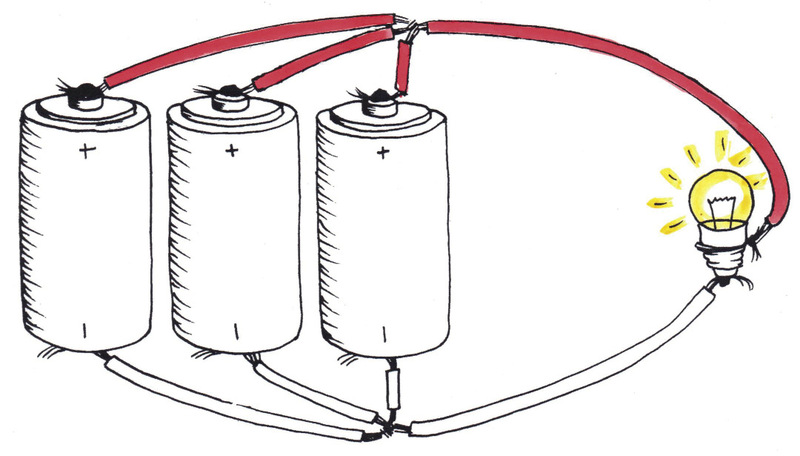
In Figures 11 and 12, each bulb gets1,5 V. Therefore, the bulbs in these figures glow brighter than those in Figure 9 (0,75 V per bulb), but dimmer than those in Figure 10 (2,25 V per bulb).
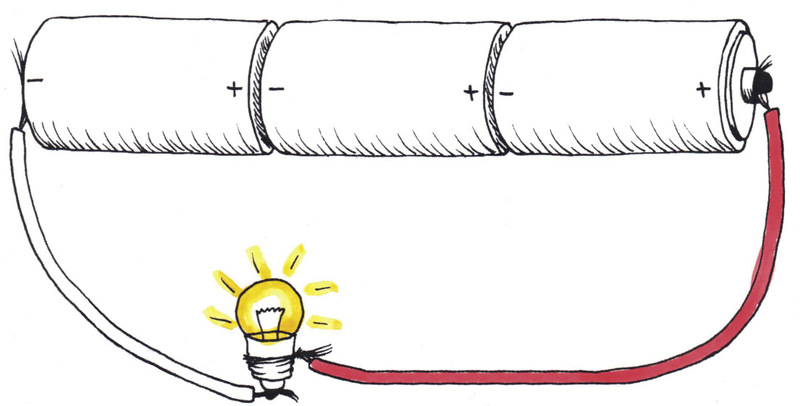
1. Compare the circuits in Figures 12 and 13. Each circuit has three cells and one light bulb, but the components are connected differently.
(a) In which circuit will the light bulb glow the brightest? Explain your answer.
(b) In which circuit will the light bulb glow for the longest amount of time before the cells are "dead" or "flat"? Explain your answer.
Photovoltaic cells
This photo shows a type of energy input device that you learnt about in Chapter 7. In this device, the energy it gives does not come from reactions between chemicals. Instead, it gives energy that comes from light. This device is called a photovoltaic cell. Photovoltaic comes from "photo" meaning "light" and "voltaic" meaning you can get volts from the device.
The black parts in this picture are a special substance called a semiconductor. This semi-conductor substance is made of thin layers, like sheets of plastic laid on top of each other. When light hits these special layers, the energy of the light is given to electric charges in the layers. The positive charges gather on one side and the negative charges on the other side.

When charges are separated like this, there is potential energy between them. If you connect wires to the positive and negative sides, the charges will flow through an output device such as a bulb, beeper or motor.
People often store the electrical energy generated by photovoltaic cells in a rechargeable electrochemical battery. The photovoltaic cells generate electrical energy during the day when the sun shines, and this energy is then stored in the rechargeable battery. When it is dark at night, the photovoltaic cells do not generate any electrical energy. But then people can use the electrical energy stored in the rechargeable battery to power lights and other devices.
Where we use photovoltaic batteries
Perhaps someone in the class has a calculator that has a little photovoltaic battery. When you hold the photovoltaic cell in sunlight, the calculator can switch on. It will work even if you move it into the shade since it has a little battery that stores the energy.
Photovoltaic cells can be very big. They can be big enough to cover the roof of a house.
The house then gets its electricity from sunlight. You might also see photovoltaic cells outside a shop where you go to recharge your cell phone.

1. Why do you think the shop has photovoltaic cells outside instead of inside?
2. On which side of the roof of a house will you put photovoltaic cells? Why?
What have you learnt?
1. Complete the following sentence: A____________reaction inside an electrochemical cell produces____________energy
2. What kind of cell does not use chemical reactions to produce energy?
3. When you connect the terminals of cells in series, you connect positive to negative to positive to negative, and so on. The cells don't have to lie head to tail. They can lie next to each other. Draw wires between the terminals of these cells to show how you would connect them in series.
|
|
4. If you connect the three 1,5 V cells in series, what voltage will the battery give you?
5. Draw wires between the terminals of these cells to show how you would connect them in parallel.
6. If you connect the three 1,5 V cells in parallel, what voltage will the battery give you?
|
|
Something you could try at home
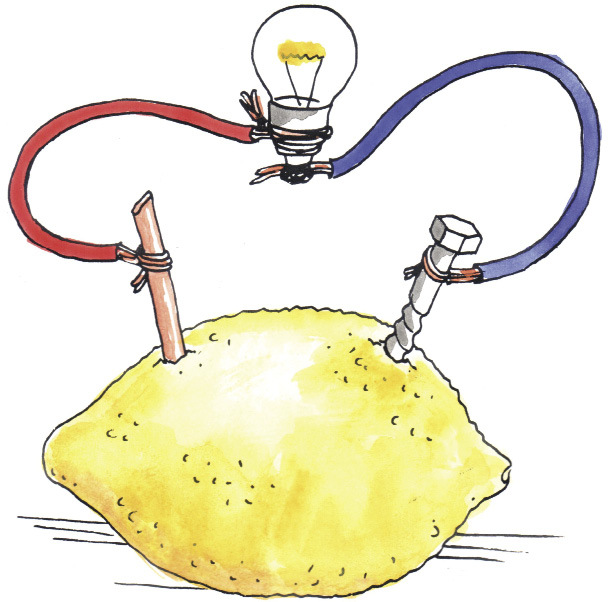
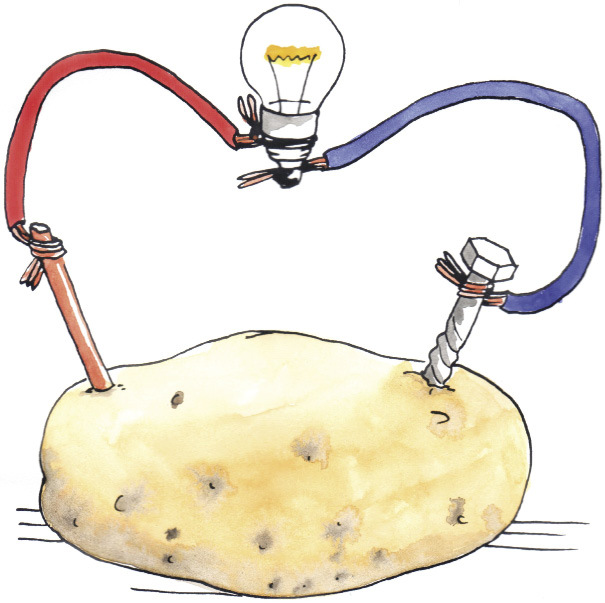
You can make a cell using a lemon or a potato, with a piece of zinc-coated metal and a piece of copper. These cells work in the same way as the cell you made in Figure 5. The lemon or the potato plays the same role as the piece of cloth or cardboard soaked in salty water. They are electrolytes through which certain small ions can move to complete the circuit. They are also membranes that prevent other, bigger ion, such as the metal ion, from moving from one electrode to the other.
Next week
Next week, you will learn how electricity is generated and distributed around the country, and about the environmental and social impact of electricity generation.
Read about where electricity comes from in Grade 8 Book 1 Chapter 10, on pages 141 to 148. The environmental impact of burning coal and other fuels is also discussed there.